 Born in Kent Town, Adelaide, Australia, Mark Oliphant was a Physicist, who received the prestigious Hughes Medal (other recipients include Alexander Graham Bell, Enrico Fermi, Stephen Hawking, and Andre Geim). He was also a life-long vegetarian after seeing a pig slaughtered at a farm as a child. Oliphant initially wanted to be a chemist or to practice medicine but, he says, his physics teacher, Dr Roy Burdon, “who weaned me away... from my ideas of being a chemist or a doctor and taught me the extraordinary exhilaration there was in even minor discoveries in the field of physics.” (1) It was, however, the New Zealand physicist, Ernest Rutherford who, he says, “has influenced me to the greatest extent in [my] life” (2) and that he was “was the most inspiring man I have ever met” (3). After Rutherford’s speech about the work taking place at the Cavendish Library, Cambridge, England, Oliphant “just immediately decided that this was the man I was going to work with, if possible.” (4) In 1927, after winning the ‘1851 Exhibitioner’ scholarship, he was able to do just that, as he went to study under Rutherford at Cambridge. In 1929, Oliphant gained his PhD in nuclear physics, specifically looking at the artificial disintegration of the atomic nucleus, and investigating positive ions. As the Cavendish Laboratory received significantly less funding than its findings deserved, laboratory equipment tended to be constructed from unconventional resources. One example of this was the “famed "string and ceiling wax" approach ... which included the use of biscuit and coffee tins as essential pieces of apparatus”. (5) Some of the amazing research taking place in the 1930’s included the splitting of the atom with the first ever high-powered particle accelerator, by Sir John Cockcroft and Ernest Walton, and the discoveries of both the Neutron (Sir James Chadwick) and confirmation of the existence of the Positron and the opposing spiral traces present at the production of a positron/electron pair (Patrick Blackett). It was Rutherford’s request that Oliphant investigate further the discoveries of Cockroft and Walton that lead to the discovery of the Helium-3 isotope. Oliphant says of this work that he and Rutherford “were able to discover two new kinds of atomic species, one was hydrogen of mass 3 [Tritium], unknown until that time, and the other helium of mass 3, also unknown. These new atoms were produced as a result of atomic transformations induced by our ion beam hitting targets of lithium, beryllium and other materials.” (6) The second discovery made from this work was that they “were able to show that heavy hydrogen nuclei, that is to say the cores of heavy hydrogen atoms, could be made to react with one another to produce a good deal of energy and new kinds of atoms. This particular reaction, which we discovered at this time, is the basic reaction in the so-called hydrogen bomb,” (7) athough at the time they had “no idea whatever that this would one day be applied to make hydrogen bombs. Our curiosity was just curiosity about the structure of the nucleus of the atom, and the discovery of these reactions was purely, as the Americans would put it, coincidental.” (8) It was this discovery, and his subsequent work at the University of Birmingham, which steered Oliphant towards the Manhattan Project and his work on Uranium with Ernest Lawrence (he did not work directly with Oppenheimer). He was a vociferous advocate for the peaceful proliferation of atomic energy, but early on he realised that “anybody who has a nuclear reactor can extract the plutonium from the reactor and make nuclear weapons, so that a country which has a nuclear reactor can, at any moment that it wants to, become a nuclear weapons power. And I, right from the beginning, have been terribly worried by the existence of nuclear weapons and very much against their use.” (9) After the war, Oliphant returned to Birmingham. Later, he was invited to the Australian National University, from which he established the Australian Academy of Sciences. In 1954, on her first royal visit, Queen Elizabeth II was presented with a charter from the Academy, marking its official establishment. His last major public role came as State governor of South Australia in 1971. Bibliography: (1) (Conversation with Sir Mark Oliphant, July 1967, National Library Collection, Tape 276, p. 1 of 12 page transcript (Interviewed by Hazel de Berg)).
(2) (Moyal, Ann, Portraits in Science, National Library of Australia, 1994, p. 37). (3) (Conversation with Sir Mark Oliphant, 24 July 1967, National Library Collection, Tape 276, pp. 1 & 4 of 12 page transcript (Interviewed by Hazel de Berg)). (4) (Moyal, Ann, Portraits in Science, National Library of Australia, 1994, p. 37). (5) (Cockburn, Stewart & Ellyard, David, Oliphant: the life and times of Sir Mark Oliphant, Axiom Books, Adelaide, 1981, p. 37) (6) (Conversation with Sir Mark Oliphant, 24 July 1967, National Library Collection, Tape 276, p. 5 of 12 page transcript (Interviewed by Hazel de Berg)). (7) (Conversation with Sir Mark Oliphant, 24 July 1967, National Library Collection, Tape 276, p. 5 of 12 page transcript (Interviewed by Hazel de Berg)). (8) Conversation with Sir Mark Oliphant, 24 July 1967, National Library Collection, Tape 276, p. 5 of 12 page transcript (Interviewed by Hazel de Berg). (9) Moyal, Ann, Portraits in Science, National Library of Australia, 1994, p. 31.
0 Comments
 Helium Helium Helium. The second most abundant element in the Universe (Hydrogen being the first), with many interesting properties, yet most people know of it in relation to balloons and a squeaky voice. But there is so much more to this tiny little element. At room temperature, Helium (He) is a gas. Actually, that would be the case in a room built anywhere on the surface of the Earth, even one built at Vostok in Antarctica. That is because its boiling point is −268.93 °C, −452.07 °F, 4.22K (K = Kelvin, the temperature scale that starts at absolute zero. All further temperatures in this article will be quoted in Kelvin). Helium is also the only element that cannot be solidified by reducing the temperature alone, needing 25 Bar of pressure to force it to solidify. Normal sea-level pressure is 1.01325 bar Even this solid, however, will melt at temperatures of about 1K. 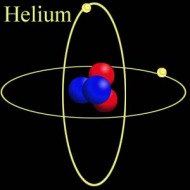 Structure of Helium-4 Structure of Helium-4 Mass and Reactivity. All forms of Helium have 2 protons in the nucleus (the centre) of the atom. In Scientific terms, this is noted as having an Atomic Number of 2 (that is the meaning of the big "2" in the picture above). In the picture on the left, the two red globes represent the protons. The most common isotope (or type) of Helium, Helium-4, has two neutrons in the Nucleus as well as two protons. The blue globes in the picture on the right represent the neutrons. Thus its Atomic Weight is approximately 4 (it is actually 4.002602, rounded up to 4.003 in the picture above). The only other stable isotope is Helium-3, which has two protons and one neutron. Second only to Neon, Helium is the most inert, or reactive element known. All of the elements in the far right column of the periodic table are called the Noble or Inert gases, as they have a complete electron configuration, and it is this which leads to the lack of reactive chemistry in the group (though the heavier elements, such as Krypton, have an extensive chemistry). The electrons for Helium are shown as small gold globes orbiting the nucleus in the picture above-right. 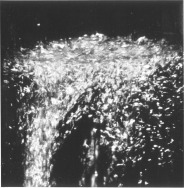 The Superfluid Transition. Liquid Helium is different from most other liquids in that it has two distinct phases. The first phase, known as Helium I, is for the most part a normal liquid (albeit a very cold one). However, the second phase, known as Helium II, has many interesting properties. Helium-4 (the heavier isotope) undergoes a phase transition from Helium I to Helium II at about 2.17K. This temperature is called the 'Lambda Point' (because the graph of the specific heat capacity as a function of the temperature resembles the Greek letter Lambda). Once the Lambda point has been navigated, the phase called Helium II is reached. The transition is marked by an intense bubbling and boiling of the liquid. This occurs because there is a sharp increase in the specific heat capacity. After this point, the liquid becomes completely calm. It is now a superfluid. Click here to see a flim which shows the Lambda Point transition. The transition itself occurs between 6:02 and 6:14.  The Effect of Zero Viscosity. Liquid Helium II has the ability to flow through the tiny holes in a porous vessel. The more the liquid is cooled below the Lambda point, the faster it will flow through the nano-capillaries. This is why Helium II has to be kept in a non-porous vessel. The reason this unique ability exists is that liquid Helium II has zero viscosity in some measurable situations. Viscosity is how resistance to stress is measured in a fluid. This effectively means viscosity is the thinkness of the fluid. Marmite is very thick, water is much thinner, and a superfluid like Helium II is very thin. It is for this reason that holes which would not admit water, would allow the Helium II to pass through unhindered, just as some holes that admit water, block the path of Marmite. Interestingly, the liquid that escapes through the porous vessel is actually colder than the remaining liquid. Click here for a video showing the escape of Helium II from a propus vessel. 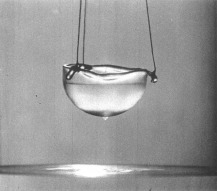 The Rollin Film. Liquid Heluim II also has the ability to move against gravity and escape from the vessel in which it is being held. It does this by forming a very thin (30 nm thick) film which creeps along the sides of the container, until it finds a warmer area, when it evaporates. This is also known as the creeping effect. The thin film is called the Rollin film, named after the man who first noticed the effect, Bernard V. Rollin. A video of this effect can be seen here.  The Fountain Effect. To produce the Fountain Effect, where a continuous flow of Helium can be seen flowing against gravity in the form of a fountain, the following needs to be done. Firstly, a small heater needs to be installed into a tube with a wide opening at one end and a very small opening at the other. The wide end of the tube needs to be blocked with a porous plug. Next. the tube should be inserted into a bath of Helium II, with the large blocked end below the surface. Once heat has been applied to the heater, the pressure builds up in the tube, and a fountain of Helium II spouts from the small opening at the top of the tube. Click here to see a video of the Fountain Effect. 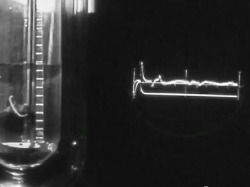 Second Sound. In Helium II, heat is carried by waves, rather than by the normal process of diffusion. This is slightly more difficut to describe in non-scientific terms without illustration, so I shall leave you with the video for this one. In part two, I will discuss the uses and the collection of Helium-3. This playlist from YouTube has all of the videos linked above in order, if you want to see the whole thing:http://www.youtube.com/view_play_list?p=442F47F12D99C4D2 |
Categories
All
Archives
November 2013
|
MOST VIEWED POSTS
© James Edward Hughes 2013
 RSS Feed
RSS Feed





