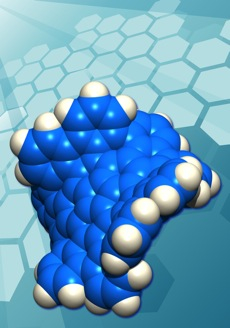
 Scientists have synthesised a new form of Carbon, containing 80 interlocking rings of carbon atoms, five of which are seven-atom carbon rings, and one a five-atom carbon ring. The odd-numbered rings bend the usually planar orientation of graphene-like substances, warping the new substance away from the usual flat, sheet-like appearance. By adding the odd-numbered rings, many properties appear changed with respect to graphene. There is a difference in colour, electrical conductivity, and solubility. It also exhibits chirality. This compound demonstrates that the electrical properties of graphine can be modified in a predictable manner. The diagram below, taken from the paper published in Nature Magazine, shows how the planarity is warped by the introduction of the odd-numbered carbon rings.
0 Comments
Finally, elements 114 and 116 have been formally added to the Periodic Table. They were officially recognised by a working group from the International Union of Pure and Applied Chemistry (IUPAC), after the results from the original experements were successfully replicated. Element 114 (ununquadium) was discovered in December 1998, when isotopes of Plutonium and Calcium, provided by the Lawrence Livermore National Laboratory in the USA, were fused by scientists at the joint Institute for Nuclear Research in Russia (Dubna). The results were made public in 1999, and at the time only one atom had been identified. Element 116 (Ununhexium) was discovered at the same research facility, fusing the elements Curium and Calcium. The working group from IUPAC has released a paper, published in the Journal of Applied Chemistry, looking at the evidence for the existence of all the elements from 113 to 118. It is in the cases of 114 and 116 that the evidence is "beyond resonable doubt", and hence they have now been included in the Periodic Table. You can read the paper by clicking here. The next stage is to decide on the prober names of the two elements. Russian scientists are reported to be considering “flerovium” for 114, after the scientist Georgy Flyorov, and “moscovium” for 116 after the capital, Moscow. Below is an excellent video from Periodic Videos detailing the full story.  Born in Kent Town, Adelaide, Australia, Mark Oliphant was a Physicist, who received the prestigious Hughes Medal (other recipients include Alexander Graham Bell, Enrico Fermi, Stephen Hawking, and Andre Geim). He was also a life-long vegetarian after seeing a pig slaughtered at a farm as a child. Oliphant initially wanted to be a chemist or to practice medicine but, he says, his physics teacher, Dr Roy Burdon, “who weaned me away... from my ideas of being a chemist or a doctor and taught me the extraordinary exhilaration there was in even minor discoveries in the field of physics.” (1) It was, however, the New Zealand physicist, Ernest Rutherford who, he says, “has influenced me to the greatest extent in [my] life” (2) and that he was “was the most inspiring man I have ever met” (3). After Rutherford’s speech about the work taking place at the Cavendish Library, Cambridge, England, Oliphant “just immediately decided that this was the man I was going to work with, if possible.” (4) In 1927, after winning the ‘1851 Exhibitioner’ scholarship, he was able to do just that, as he went to study under Rutherford at Cambridge. In 1929, Oliphant gained his PhD in nuclear physics, specifically looking at the artificial disintegration of the atomic nucleus, and investigating positive ions. As the Cavendish Laboratory received significantly less funding than its findings deserved, laboratory equipment tended to be constructed from unconventional resources. One example of this was the “famed "string and ceiling wax" approach ... which included the use of biscuit and coffee tins as essential pieces of apparatus”. (5) Some of the amazing research taking place in the 1930’s included the splitting of the atom with the first ever high-powered particle accelerator, by Sir John Cockcroft and Ernest Walton, and the discoveries of both the Neutron (Sir James Chadwick) and confirmation of the existence of the Positron and the opposing spiral traces present at the production of a positron/electron pair (Patrick Blackett). It was Rutherford’s request that Oliphant investigate further the discoveries of Cockroft and Walton that lead to the discovery of the Helium-3 isotope. Oliphant says of this work that he and Rutherford “were able to discover two new kinds of atomic species, one was hydrogen of mass 3 [Tritium], unknown until that time, and the other helium of mass 3, also unknown. These new atoms were produced as a result of atomic transformations induced by our ion beam hitting targets of lithium, beryllium and other materials.” (6) The second discovery made from this work was that they “were able to show that heavy hydrogen nuclei, that is to say the cores of heavy hydrogen atoms, could be made to react with one another to produce a good deal of energy and new kinds of atoms. This particular reaction, which we discovered at this time, is the basic reaction in the so-called hydrogen bomb,” (7) athough at the time they had “no idea whatever that this would one day be applied to make hydrogen bombs. Our curiosity was just curiosity about the structure of the nucleus of the atom, and the discovery of these reactions was purely, as the Americans would put it, coincidental.” (8) It was this discovery, and his subsequent work at the University of Birmingham, which steered Oliphant towards the Manhattan Project and his work on Uranium with Ernest Lawrence (he did not work directly with Oppenheimer). He was a vociferous advocate for the peaceful proliferation of atomic energy, but early on he realised that “anybody who has a nuclear reactor can extract the plutonium from the reactor and make nuclear weapons, so that a country which has a nuclear reactor can, at any moment that it wants to, become a nuclear weapons power. And I, right from the beginning, have been terribly worried by the existence of nuclear weapons and very much against their use.” (9) After the war, Oliphant returned to Birmingham. Later, he was invited to the Australian National University, from which he established the Australian Academy of Sciences. In 1954, on her first royal visit, Queen Elizabeth II was presented with a charter from the Academy, marking its official establishment. His last major public role came as State governor of South Australia in 1971. Bibliography: (1) (Conversation with Sir Mark Oliphant, July 1967, National Library Collection, Tape 276, p. 1 of 12 page transcript (Interviewed by Hazel de Berg)).
(2) (Moyal, Ann, Portraits in Science, National Library of Australia, 1994, p. 37). (3) (Conversation with Sir Mark Oliphant, 24 July 1967, National Library Collection, Tape 276, pp. 1 & 4 of 12 page transcript (Interviewed by Hazel de Berg)). (4) (Moyal, Ann, Portraits in Science, National Library of Australia, 1994, p. 37). (5) (Cockburn, Stewart & Ellyard, David, Oliphant: the life and times of Sir Mark Oliphant, Axiom Books, Adelaide, 1981, p. 37) (6) (Conversation with Sir Mark Oliphant, 24 July 1967, National Library Collection, Tape 276, p. 5 of 12 page transcript (Interviewed by Hazel de Berg)). (7) (Conversation with Sir Mark Oliphant, 24 July 1967, National Library Collection, Tape 276, p. 5 of 12 page transcript (Interviewed by Hazel de Berg)). (8) Conversation with Sir Mark Oliphant, 24 July 1967, National Library Collection, Tape 276, p. 5 of 12 page transcript (Interviewed by Hazel de Berg). (9) Moyal, Ann, Portraits in Science, National Library of Australia, 1994, p. 31. |
Categories
All
Archives
November 2013
|
MOST VIEWED POSTS
© James Edward Hughes 2013
 RSS Feed
RSS Feed





